

Meteorology, Climatology, and Atmospheric Science Blood pressure units from a sphygmomanometer are in terms of millimeters of mercury (mm Hg). As cuff pressure continues to decrease, eventually sound is no longer heard this is the diastolic pressure-the lowest pressure (resting phase) in the cardiac cycle. This is followed by a decrease in pressure as the heart’s ventricles prepare for another beat. When the cuff’s pressure equals the arterial systolic pressure, blood flows past the cuff, creating audible sounds that can be heard using a stethoscope. This rise in pressure at which blood flow begins is the systolic pressure-the peak pressure in the cardiac cycle. As the heart beats, blood forced through the arteries causes a rise in pressure.
When using a sphygmomanometer, the cuff is placed around the upper arm and inflated until blood flow is completely blocked, then slowly released.
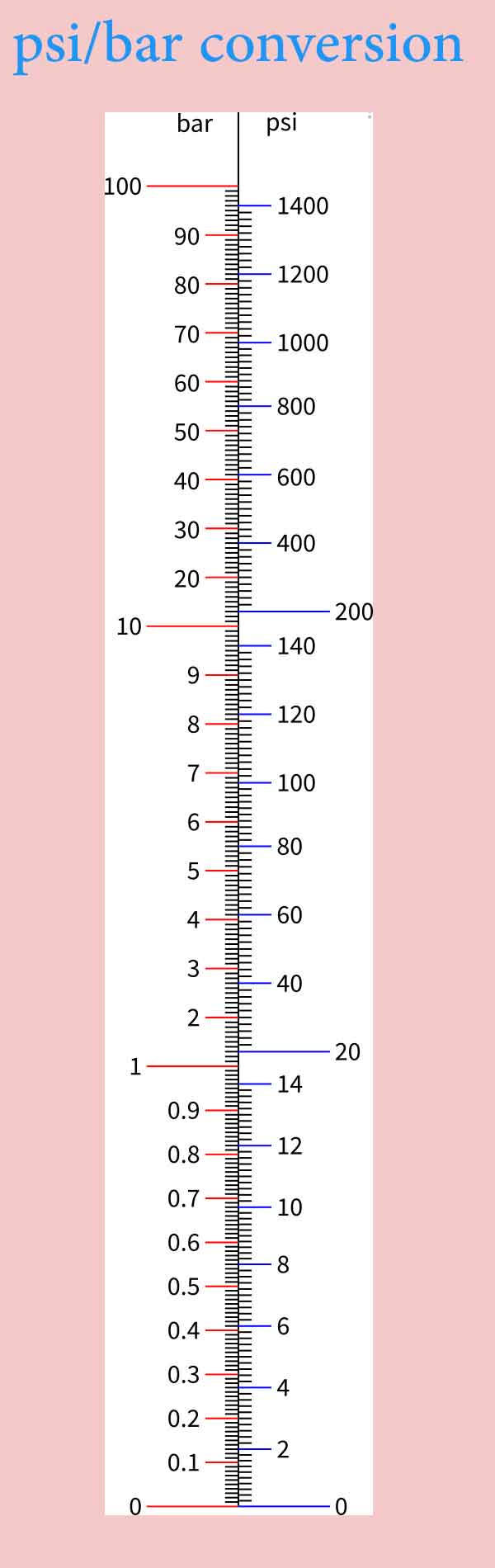
#1 bar to psi at sea level manual#
There are many types of sphygmomanometers: manual ones that require a stethoscope and are used by medical professionals mercury ones, used when the most accuracy is required less accurate mechanical ones and digital ones that can be used with little training but that have limitations. Since its invention in 1881, it has been an essential medical device. It consists of an inflatable cuff to restrict blood flow, a manometer to measure the pressure, and a method of determining when blood flow begins and when it becomes impeded ( ). Determine the pressure of the gas in:īlood pressure is measured using a device called a sphygmomanometer (Greek sphygmos = “pulse”). The pressure of a sample of gas is measured at sea level with an open-end Hg manometer, as shown to the right. In general, pressure is defined as the force exerted on a given area: \(P=\phantom\) If you actually perch a bowling ball on your thumbnail, the pressure experienced is twice the usual pressure, and the sensation is unpleasant. These may seem like huge amounts, and they are, but life on earth has evolved under such atmospheric pressure. At sea level, this pressure is roughly the same as that exerted by a full-grown African elephant standing on a doormat, or a typical bowling ball resting on your thumbnail. This can be seen by using the ideal gas law as an approximation.A dramatic illustration of atmospheric pressure is provided in this brief video, which shows a railway tanker car imploding when its internal pressure is decreased.Ī smaller scale demonstration of this phenomenon is briefly explained.Ītmospheric pressure is caused by the weight of the column of air molecules in the atmosphere above an object, such as the tanker car. Other things being equal, hotter air is less dense than cooler air and will thus rise through cooler air. Air is a mixture of gases and the calculations always simplify, to a greater or lesser extent, the properties of the mixture. ĭepending on the measuring instruments used, different sets of equations for the calculation of the density of air can be applied. Pure liquid water is 1,000 kg/m 3 (62 lb/cu ft).Īir density is a property used in many branches of science, engineering, and industry, including aeronautics gravimetric analysis the air-conditioning industry atmospheric research and meteorology agricultural engineering (modeling and tracking of Soil-Vegetation-Atmosphere-Transfer (SVAT) models) and the engineering community that deals with compressed air. At 101.325 kPa (abs) and 15 ☌ (59 ☏), air has a density of approximately 1.225 kg/m 3 (0.0765 lb/cu ft), which is about 1⁄ 800 that of water, according to the International Standard Atmosphere (ISA). At 101.325 kPa (abs) and 20 ☌ (68 ☏), air has a density of approximately 1.204 kg/m 3 (0.0752 lb/cu ft), according to the International Standard Atmosphere (ISA). It also changes with variations in atmospheric pressure, temperature and humidity. Air density, like air pressure, decreases with increasing altitude. The density of air or atmospheric density, denoted ρ, is the mass per unit volume of Earth's atmosphere. Mass per unit volume of earths atmosphere


 0 kommentar(er)
0 kommentar(er)
